 Examples of Organic Reactions Examples of Organic Reactions
 1. Ionic Reactions 1. Ionic Reactions
 The principles and terms introduced in the previous sections can now be summarized and illustrated by the following three examples. Reactions such as these are called ionic or polar reactions, because they often involve charged species and the bonding together of electrophiles and nucleophiles. Ionic reactions normally take place in liquid solutions, where solvent molecules assist the formation of charged intermediates. The principles and terms introduced in the previous sections can now be summarized and illustrated by the following three examples. Reactions such as these are called ionic or polar reactions, because they often involve charged species and the bonding together of electrophiles and nucleophiles. Ionic reactions normally take place in liquid solutions, where solvent molecules assist the formation of charged intermediates.
 |
 The substitution reaction shown on the left can be viewed as taking place in three steps. The first is an acid-base equilibrium, in which HCl protonates the oxygen atom of the alcohol. The resulting conjugate acid then loses water in a second step to give a carbocation intermediate. Finally, this electrophile combines with the chloride anion nucleophile to give the final product. The substitution reaction shown on the left can be viewed as taking place in three steps. The first is an acid-base equilibrium, in which HCl protonates the oxygen atom of the alcohol. The resulting conjugate acid then loses water in a second step to give a carbocation intermediate. Finally, this electrophile combines with the chloride anion nucleophile to give the final product.
|
|
 The addition reaction shown on the left can be viewed as taking place in two steps. The first step can again be considered an acid-base equilibrium, with the pi-electrons of the carbon-carbon double bond functioning as a base. The resulting conjugate acid is a carbocation, and this electrophile combines with the nucleophilic bromide anion. The addition reaction shown on the left can be viewed as taking place in two steps. The first step can again be considered an acid-base equilibrium, with the pi-electrons of the carbon-carbon double bond functioning as a base. The resulting conjugate acid is a carbocation, and this electrophile combines with the nucleophilic bromide anion.
|
|
 The elimination reaction shown on the left takes place in one step. The bond breaking and making operations that take place in this step are described by the curved arrows. The initial stage may also be viewed as an acid-base interaction, with hydroxide ion serving as the base and a hydrogen atom component of the alkyl chloride as an acid. The elimination reaction shown on the left takes place in one step. The bond breaking and making operations that take place in this step are described by the curved arrows. The initial stage may also be viewed as an acid-base interaction, with hydroxide ion serving as the base and a hydrogen atom component of the alkyl chloride as an acid.
|
|
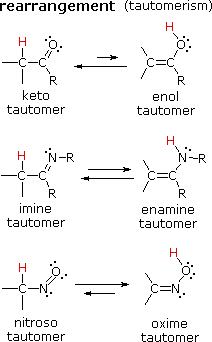 |
 There are many kinds of molecular rearrangements. The examples shown on the left are from an important class called tautomerization or, more specifically, keto-enol tautomerization. There are many kinds of molecular rearrangements. The examples shown on the left are from an important class called tautomerization or, more specifically, keto-enol tautomerization.
 Tautomers are rapidly interconverted constitutional isomers, usually distinguished by a different bonding location for a labile hydrogen atom (colored red here) and a differently located double bond. Tautomers are rapidly interconverted constitutional isomers, usually distinguished by a different bonding location for a labile hydrogen atom (colored red here) and a differently located double bond.
 The equilibrium between tautomers is not only rapid under normal conditions, but it often strongly favors one of the isomers (acetone, for example, is 99.999% keto tautomer). Even in such one-sided equilibria, evidence for the presence of the minor tautomer comes from the chemical behavior of the compound. The equilibrium between tautomers is not only rapid under normal conditions, but it often strongly favors one of the isomers (acetone, for example, is 99.999% keto tautomer). Even in such one-sided equilibria, evidence for the presence of the minor tautomer comes from the chemical behavior of the compound.
 Tautomeric equilibria are catalyzed by traces of acids or bases that are generally present in most chemical samples. Tautomeric equilibria are catalyzed by traces of acids or bases that are generally present in most chemical samples.
|
 2. Radical Reactions 2. Radical Reactions
 If methane gas is mixed with chlorine gas and exposed to sunlight an explosive reaction takes place in which chlorinated methane products are produced along with hydrogen chloride. An unbalanced equation illustrating this reaction is shown below; the relative amounts of the various products depends on the proportion of the two reactants that are used. If methane gas is mixed with chlorine gas and exposed to sunlight an explosive reaction takes place in which chlorinated methane products are produced along with hydrogen chloride. An unbalanced equation illustrating this reaction is shown below; the relative amounts of the various products depends on the proportion of the two reactants that are used.
 How does this reaction take place? Gas phase reactions, such as the chlorination of methane, do not normally proceed via ionic intermediates. Strong evidence indicates that neutral radical intermediates, sometimes called free radicals, play a role in this and many other similar transformations. How does this reaction take place? Gas phase reactions, such as the chlorination of methane, do not normally proceed via ionic intermediates. Strong evidence indicates that neutral radical intermediates, sometimes called free radicals, play a role in this and many other similar transformations.
 A radical is an atomic or molecular species having an unpaired, or odd, electron. Some radicals, such as nitrogen dioxide (NO2) and nitric oxide (NO) are relatively stable, but most are so reactive that isolation and long-term study under normal conditions is not possible. A radical is an atomic or molecular species having an unpaired, or odd, electron. Some radicals, such as nitrogen dioxide (NO2) and nitric oxide (NO) are relatively stable, but most are so reactive that isolation and long-term study under normal conditions is not possible.
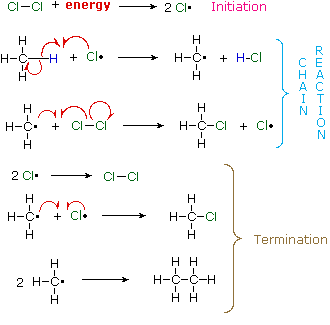
 A set of radical reactions called a chain reaction can account for all the facts observed for this process. A set of radical reactions called a chain reaction can account for all the facts observed for this process.
 |
|
 The reaction is initiated by the input of energy (heat or light). The weak chlorine-chlorine bond is broken homolytically to give chlorine atoms. The reaction is initiated by the input of energy (heat or light). The weak chlorine-chlorine bond is broken homolytically to give chlorine atoms.
|
|
 In these two reactions radical intermediates abstract an atom from one of the reactant molecules. If a chlorine atom abstracts a hydrogen from methane in the first step, the resulting methyl radical abstracts a chlorine atom from chlorine in the second step, regenerating a chlorine atom. This is therefore a chain reaction. In these two reactions radical intermediates abstract an atom from one of the reactant molecules. If a chlorine atom abstracts a hydrogen from methane in the first step, the resulting methyl radical abstracts a chlorine atom from chlorine in the second step, regenerating a chlorine atom. This is therefore a chain reaction.
|
|
 In principle a chain reaction should continue until one or both of the reactants are consumed. In practice, however, such reactions stop before completion and have to be reinitiated. In principle a chain reaction should continue until one or both of the reactants are consumed. In practice, however, such reactions stop before completion and have to be reinitiated.
 This happens whenever two radical intermediates meet and combine to give a stable molecule, thus terminating the chain of reactions. This happens whenever two radical intermediates meet and combine to give a stable molecule, thus terminating the chain of reactions.
 Since radical intermediates are extremely reactive and are present in very low concentration, the probability that two such intermediates will collide is small. Consequently, the chain reaction will proceed through many cycles before termination occurs. Since radical intermediates are extremely reactive and are present in very low concentration, the probability that two such intermediates will collide is small. Consequently, the chain reaction will proceed through many cycles before termination occurs.
|
|